Boyle's Law
In
1662, Robert Boyle (British chemist 1627 - 1691) studied
the relationship between the pressure and the volume of a gas at a constant
temperature.
Boyle observed that the product of the
pressure and volume are observed to be nearly constant. The product of pressure
and volume is exactly a constant for an ideal gas.
P X V = constant
For
2 different gases Boyle’s law formula
P1
X V1 = P2 X V2
P1, V1 (pressure and volume for the
first gas).
P2, V2 (pressure and volume for the
second gas).
Boyle’s experiment:
Robert Boyle used a sealed end J-shaped
piece of glass tubing, a gas (air) was trapped in the sealed end of the tube
and varying amounts of mercury were added to the J-shaped tube to vary the
pressure of the system. Boyle systematically varied the pressure and measured
the volume of the gas. These measurements were performed using a fixed amount
of gas at constant temperature. In this way Boyle was able to examine the
pressure-volume relationship without complications from other factors such as
changes in temperature or amount of gas.
Volume
of a fixed mass of gas at a constant temperature is inversely proportional to
the pressure of the gas.
When
volume increases, pressure decreases, and vise versa
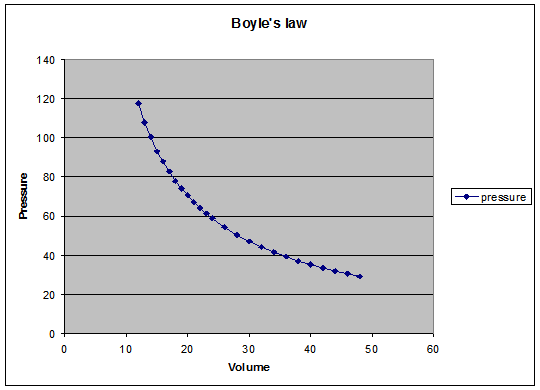
Graph the given data at http://webserver.lemoyne.edu/faculty/giunta/
And use the
equation PXV= constant will give the
results as below:
|
volume |
Pressure PXV |
|
48 |
29.125 1398 |
|
46 |
30.5625 1405.8 |
|
44 |
31.9375 1405.25 |
|
42 |
33.5 1407 |
|
40 |
35.3125 141.25 |
|
38 |
37 1406 |
|
36 |
39.3125 141525 |
|
34 |
41.625 1415.25 |
|
32 |
44.1875 1414 |
|
30 |
47.0625 1411.87 |
|
28 |
50.3125 1408.75 |
|
26 |
54.3125 1412.19 |
|
24 |
58.8125 1411.56 |
|
23 |
61.3125 1410.1 |
|
22 |
64.0625 1409.37 |
|
21 |
67.0625 1408.31 |
|
20 |
70.6875 1413.75 |
|
19 |
74.125 1408.37 |
|
18 |
77.875 1401.75 |
|
17 |
82.75 1406.75 |
|
16 |
87.875 1406.01 |
|
15 |
93.0625 1395.97 |
|
14 |
100.438 1406.13 |
|
13 |
107.813 1401.56 |
|
12 |
117.563 1410.36 |
Conclusion
Graph type shows an inverse proportional
between pressure and volume, also by using the given data and applying them
using Boyle’s equation P X V =
constant
we
always get a constant, even though it’s not the exact same number and this
occurred because we didn’t use an ideal gas here (A gas consider ideal when its
particles has a negligible volume, with no intermolecular forces and its atoms or molecules
undergo perfectly elastic collisions).